Specimen Collection Manual: Nasopharyngeal for Eosinophils
Eosinophils and PMNs Smear
Instructions
Materials/Supplies
- A dry, flexible, type 1 aluminum-shafted, Rayon- or Dacron-tipped wire swab with culture/transport media should be used for this procedure.
- Use separate swabs when collecting specimens from both nostrils.
Specimen Collection Criteria
- Insert the swab into one nostril.
- The swab tip should touch the mucosal surface of the mid-inferior portion of the inferior turbinate:
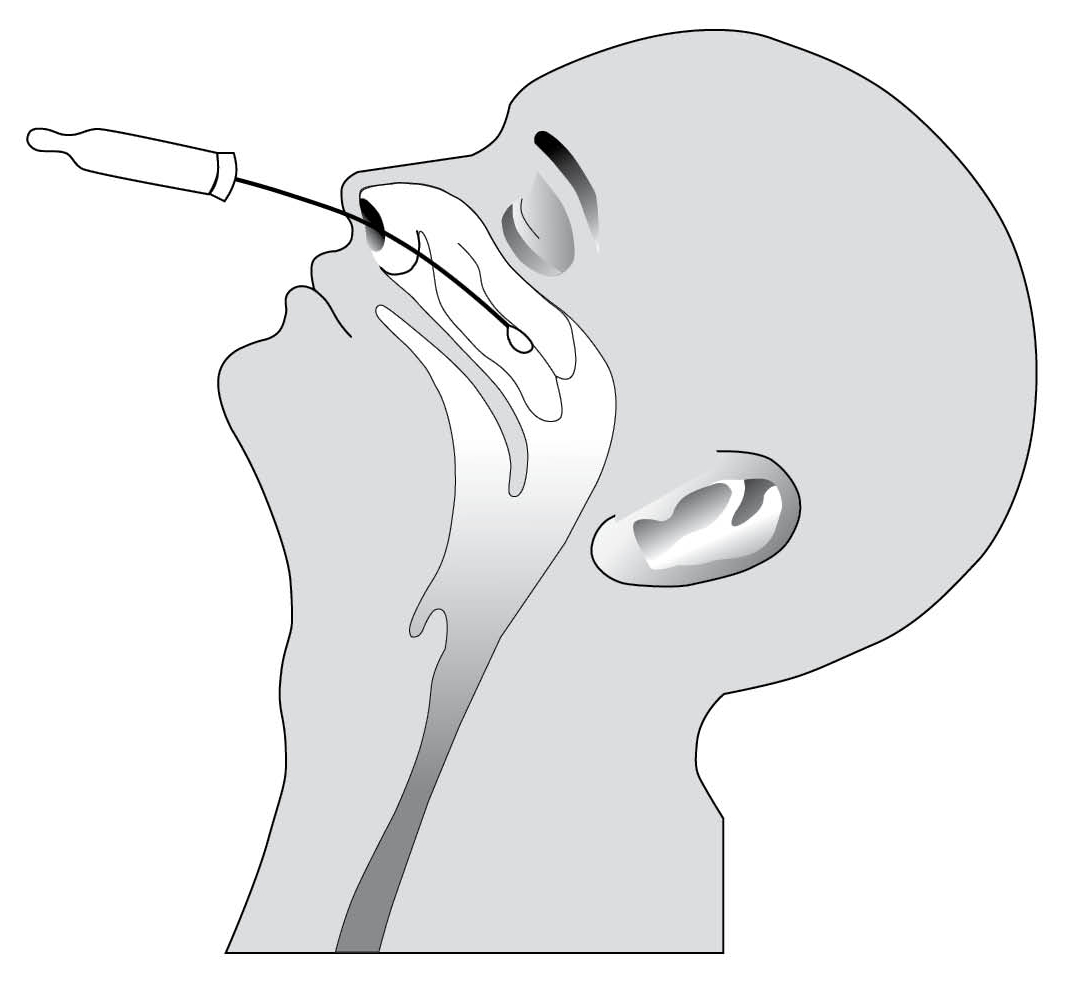
- Hold the swab in place for 5-15 seconds, then gently rotate the swab three times to loosen and collect cellular material.
- Withdraw the swab and rub it on the surface of an acetone-cleaned slide. Be sure to roll the swab so that the entire surface of the swab comes into contact with the slide.
- Repeat the process on a second slide.
- Allow the slides to air dry.
- Send the slides to the Laboratory in a slide mailer.
- If submitting a swab only, ensure insertion of the swab into transport medium is performed so that the swab becomes moistened.
Rejection Criteria
- Dry culturettes and dry swabs.
Reference
Applicable Tests:
Contacts
Hematology/Urinalysis Laboratory – GP
313-473-1809
Name: Hematology/Urinalysis Laboratory – GP
Location:
Phone: 313-473-1809
Hematology Laboratory – RO
248-551-8080
Name: Hematology Laboratory – RO
Location:
Phone: 248-551-8080
Hematology/Coagulation Laboratory – TR
248-964-8040
Name: Hematology/Coagulation Laboratory – TR
Location:
Phone: 248-964-8040
Last Updated
5/24/2024
Microtainer® and Vacutainer® are registered trademarks of Becton, Dickinson and Company.
UroVysion® is a registered trademark of Abbott Laboratories. ThinPrep® is a registered trademark of Hologic, Incorporated.