Occult Blood, FIT Diagnostic, Stool
Polymedco Fecal Immunological Test (FIT), Colorectal Cancer Screening, FIT Test, Fecal Immunochemical Test Sampling Bottle
Test Codes
EPIC: LAB1230898, Beaker: OBFIT
Department
Urinalysis
Instructions
- This test is available for outpatients only.
- Fecal Immunochemical Test Sampling Bottle with return mailers may be obtained from Beaumont Laboratory Client Services at 800-551-0488.
- Physician office completes the required information on the yellow Patient Identification Card prior to distribution. This includes Name, Date of Birth, Patient’s Address, Patient’s Phone Number, and Physician.
Specimen Collection Criteria
Collect: Fresh, random, stool specimen, collected as described below:
- Obtain the appropriate Fecal Immunochemical Test Sampling Bottle.
- Patient completes the Sample Collection Date on the yellow Patient Identification Card.
- Patient completes the required information on the yellow card provided - this includes Name, Date of Birth, Patient's Address, Patients' Phone Number, Patient's Physician, and Sample Collection Date.
- Patient collects the stool per sample collection instructions provided in the Package Insert for Personal Use Kit. NOTE: Collection paper is supplied to deposit the stool sample. Contamination from toilet water must be avoided.
- Patient dips the sample probe into the fresh stool specimen. The stool sample must fill the grooved portion of the probe.
- Patient inserts the sample probe into the Sampling Bottle and snaps the green cap on tightly to close the bottle.
- Patient returns the Sampling Bottle and yellow card to his/her physician in the Beaumont Laboratory return mailer envelope provided for Outreach Courier pickup, OR mails directly to Beaumont Laboratory via US Postal Service.
- Specimen must be returned immediately after collection (within three days).
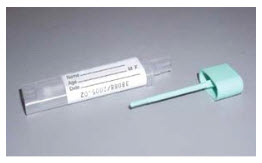
Physician Office/Draw Specimen Preparation
Maintain FIT at room temperature (20-26°C or 68-78.8°F) or refrigerated (2-8°C or 36-46°F) prior to transport.
Preparation for Courier Transport
Transport: FIT, at room temperature (20-26°C or 68-78.8°F) or refrigerated (2-8°C or 36-46°F).
Rejection Criteria
- Unpreserved stool specimen (specimen not received in FIT Sampling Bottle).
- Frozen specimens.
- Specimens not collected and processed as indicated.
- Sampling Bottle received without proper identification (Name, Date of Birth, Sample Collection Date).
- Sampling Bottle received greater than 15 days from sample collection date.
- Specimens received without the Patient Identification Card will not be tested if the sample cannot be identified. Samples will be held in the lab for 30 days.
In-Lab Processing
Specimens may be maintained at room temperature (20-26°C or 68-78.8°F) or refrigerated (2-8°C or 36-46°F) prior to testing.
Storage
Specimen Stability for Testing:
Room Temperature (20-26°C or 68-78.8°F): 14 days from date of sample collection.
Refrigerated (2-8°C or 36-46°F): 30 days from date of sample collection.
Frozen (-20°C/-4°F or below): Unacceptable.
Specimen Storage in Department Prior to Disposal:
FIT sampling vials are stored refrigerated (2-8°C or 36-46°F) for 3 days before disposal.
No additional stool testing other than FIT can be performed on the Sampling Bottle.
Laboratory
Royal Oak Automated Chemistry Laboratory
Performed
Sunday – Saturday, 7:00 am – 3:00 pm.
Results available within 24 hours of receipt in the Laboratory.
Reference Range
Negative.
Test Methodology
Qualitative Immunoassay.
Interpretation
- The test manufacturer recommends a 100 ng/mL hemoglobin cutoff producing a specificity of approximately 95% for the detection of lower gastrointestinal bleeding.
- Human hemoglobin A and hemoglobin S are detected by this method. Polymedco has not tested other hemoglobin variants.
Clinical Utility
The Polymedco Fecal Immunological Test (FIT) is used for colorectal cancer screening. By overcoming two major limitations of guaiac-based Hemoccult cards, FIT is likely to result in improved outpatient participation in colorectal cancer screening in the following ways:
- Patients only need to collect one stool sample versus the three samples required by guaiac-based methods.
- Because the FIT method detects human hemoglobin in stool, it avoids the dietary interferences that occur with guaiac-based methods (red meat, raw vegetables, vitamin C).
FIT does not detect upper gastrointestinal bleeding because hemoglobin is broken down during intestinal transit.
CPT Codes
G0328, 82274.
Contacts
Automated Chemistry Laboratory – RO
248-551-8065
Name: Automated Chemistry Laboratory – RO
Location:
Phone: 248-551-8065
Last Updated
12/19/2024
Microtainer® and Vacutainer® are registered trademarks of Becton, Dickinson and Company.
UroVysion® is a registered trademark of Abbott Laboratories. ThinPrep® is a registered trademark of Hologic, Incorporated.