Anti Nuclear Antibody Screening Test (ANA)
ANA, Anti-Nuclear Antibody Screening Test
Test Codes
EPIC: LAB5749, SOFT: ANA
Department
Special Chemistry
Specimen Collection Criteria
Collect: One Gold-top SST tube. (Minimum Whole Blood: 2.0 mL)
Physician Office/Draw Specimen Preparation
Let specimen clot 30-60 minutes then immediately centrifuge to separate serum from cells. Refrigerate (2-8°C or 36-46°F) the centrifuged collection tube within two hours of collection. (Minimum Serum: 0.5 mL)
Preparation for Courier Transport
Transport: Centrifuged collection tube, refrigerated (2-8°C or 36-46°F). (Minimum Serum: 0.5 mL)
Rejection Criteria
Plasma specimens.
Severely lipemic, icteric or hemolyzed specimens.
In-Lab Processing
Let specimen clot 30-60 minutes then immediately centrifuge to separate serum from cells. Room temperature is acceptable for a maximum of two hours. (Minimum Serum: 0.5 mL)
Storage
Specimen Stability for Testing:
Centrifuged SST Tubes and Microtainers® with Separator Gels
Room Temperature (20-26°C or 68-78.8°F): 6 hours
Refrigerated (2-8°C or 36-46°F): 7 days
Frozen (-20°C/-4°F or below): Unacceptable
Red-top Tubes and Microtainers® without Separator Gels
Room Temperature (20-26°C or 68-78.8°F): 2 hours
Refrigerated (2-8°C or 36-46°F): Unacceptable
Frozen (-20°C/-4°F or below): Unacceptable
Serum Specimens (Pour-Overs)
Room Temperature (20-26°C or 68-78.8°F): 6 hours
Refrigerated (2-8°C or 36-46°F): 7 days
Frozen (-20°C/-4°F or below): 3 months
Specimen Storage in Department Prior to Disposal:
Refrigerated (2-8°C or 36-46°F): 7 days
Laboratory
Royal Oak Special Testing Laboratory
Performed
Monday – Friday.
Results available within 1-3 days of specimen collection.
Reference Range
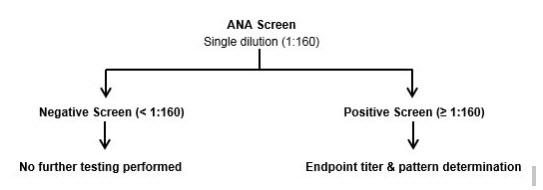
Negative (less than 1:160).
Positive ANA screens will be reflexed to titer and pattern determination.
Test Methodology
Indirect Immunofluorescent Assay (IFA).
IFA Substrate: HEp-2000.
Interpretation
Clinical Utility
- Anti-nuclear antibodies are seen in a variety of systemic rheumatic diseases. Titers of 1:40 and even 1:80 may have no clinical significance. High titers are common in SLE, while lower titers are seen in other collagen vascular disorders. The ANA incidence is 99% in SLE, 85% in Sjogren's syndrome, 88% in scleroderma, 55% in rheumatoid arthritis, and 40% in juvenile rheumatoid arthritis.
- Cytoplasmic fluorescence present on HEp-2000 cells may be due to antibodies other than ANA. Low titer positive results may occur in healthy individuals. Sera from patients undergoing successful therapy for autoimmune disorders may be negative for ANA.
- Many drugs such as hydralazine and procainamide may induce ANA.
- Homogenous staining pattern suggests anti-DNA, anti-histone, or anti-dexoyribonucleoprotein antibodies observed in SLE, RA and drug-induced SLE.
- A speckled pattern indicates (a) antibody to SSA or SSB observed in SLE and Sjogren's syndrome; (b) Smith antibody (SM) observed almost exclusively in SLE; or (c) ribonucleoprotein (RNP) antibody observed in SLE, mixed connective tissue disease, RA, and scleroderma.
- Nucleolar staining is observed in scleroderma and some forms of Raynaud's phenomenon.
- A centromere pattern is observed in the CREST syndrome of scleroderma. (CREST = Calcinosis, Raynaud's phenomenon, Esophageal dysmotility, Sclerodactyly, and Telangiectasia).
CPT Codes
86038 (Screen), 86039 (Titer).
Contacts
Special Chemistry Laboratory – RO
248-551-8044
Name: Special Chemistry Laboratory – RO
Location:
Phone: 248-551-8044
Last Updated
12/30/2022
Microtainer® and Vacutainer® are registered trademarks of Becton, Dickinson and Company.
UroVysion® is a registered trademark of Abbott Laboratories. ThinPrep® is a registered trademark of Hologic, Incorporated.